Avian Digestive System
 Prepared for the 2012 MacFarlane Pheasant Symposium
Prepared for the 2012 MacFarlane Pheasant Symposium
By: Rob Porter, DVM, PhD
Minnesota Veterinary Diagnostic Laboratory
The digestive system of birds has adaptations to facilitate flight
Many birds operate on a thin margin of metabolic safety and may require a constant source of feed to sustain activity. Birds cannot afford to store heavy food materials for long periods and digest their food rather quickly relative to mammals. In addition, the avian intestinal tract is generally shorter than that of mammals on a body weight basis. Birds lack teeth and heavy jaw muscles, which have been replaced by a light-weight beak. In gallinaceous birds, as well as others, food particles that are swallowed whole can be temporarily stored in the crop and later reduced in size by the muscular gizzard. This presentation will focus on the digestive tract of gallinaceous birds, which includes pheasants, partridges, quail, chickens and turkeys.
What is the digestive system? This is defined as the apparatus that allows the bird to prehend and swallow feed, a muscular channel (esophagus, proventriculus, gizzard, intestine) that transports the feed through the body, the salivary glands and pancreas that contribute digestive enzymes, the liver that secretes bile for fat digestion.
What is the digestive system?
This is defined as the apparatus that allows the bird to prehend and swallow feed, a muscular channel (esophagus, proventriculus, gizzard, intestine) that transports the feed through the body, the salivary glands and pancreas that contribute digestive enzymes, the liver that secretes bile for fat digestion.
Anatomy of Digestive Tract
- Beak, mouth and pharynx
- Esophagus and crop
- Proventriculus and Gizzard
- Small intestine
- Cecum
- Colon and cloaca
- Pancreas
- Liver
Digestive Enzyme Activity in the Chicken
| Location | pH | Enzyme |
|---|---|---|
| Mouth | 7.0 – 7.5 | Amylase |
| Crop | 4.5 | None – mucus secretion |
| Proventriculus + Gizzard | 2.5 | Pepsin. lipase |
| Duodenum | 6.0 | Amylase, trypsin, collagenase, bile, lipase |
| Jejunum | 5.8 – 6.8 | Maltase, sucrose, lactase, peptidases |
| Cecum | 6.8 | Microbial digestion |
Adapted from Scott’s Nutrition of the Chicken, 4th Edition. S Leeson and JD Summers. University Books, Guelph, Ontario Canada, 2001.
- Beak, tongue and pharynx
- Esophagus
- Crop
- roventriculus and Gizzard
- Small intestine
- Liver
- Pancreas
- Ileum
- Cecum
- Colon

Beak, Tongue, and Pharynx
The beak is the primary organ to prehend feed and can crush, tear or hold feed before swallowing. The upper beak (maxilla) is covered by hard keratin. Birds do not have teeth, but the margins of the beak are often hard and might have ridges to help with grasping feed. In addition, ridges (papillae) on the upper palate can help to prehend feed. Taste buds are located on the mucosa of the upper beak and behind the tongue. Chickens are reported to have less than 300 tastebuds, compared to 1700 for dogs and nearly 9000 for humans. Perhaps taste plays a smaller role in eating in birds compared to mammals. Gallinaceous birds generally do not chew, but swallow feed whole.

Tongues are covered with stiff papillae that help to used to manipulate and prehend feed. The oral cavity also contains salivary glands that secrete saliva and variable amounts of amylase; little is secreted in chickens and turkeys. Grain-eating birds have well developed salivary glands while these glands are fewer in fish and meat eaters.
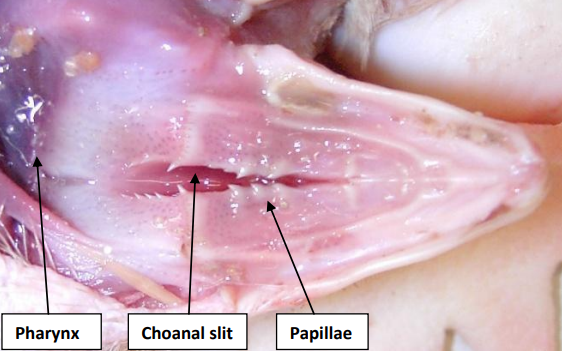
Upper Inner Beak
Upper beak of chicken: Note choanal slit, where nasal cavity communicates with oral cavity, and pointed papillae that help to prehend feed.
Esophagus and Crop
The esophagus is a muscular tube that transports feed from the pharynx to the proventriculus. Because feed is swallowed (birds rarely “chew”) whole the Pharynx Choanal slit Papillae esophagus must be quite distensible. The esophagus has a double-muscular wall (circular and longitudinal smooth muscle) and mucus glands to lubricate the channel, easing the movement of feed. Muscular contractions (peristalsis) propel the feed down the digestive tract. Gallinaceous birds have a crop, a diverticulum of the esophagus, which holds food before further digestion commences. This capacity enables the bird to take its food as “whole meals” at single time while still allowing for continuous digestion. The crop has a smooth white to pink lining (mucosa).

Proventriculus and Gizzard
The avian “stomach” consists of these two chambers. The proventriulus more closely resembles the mammalian stomach. The proventriculus is lined by compound glands that secrete mucus, pepsin and hydrochloric acid.
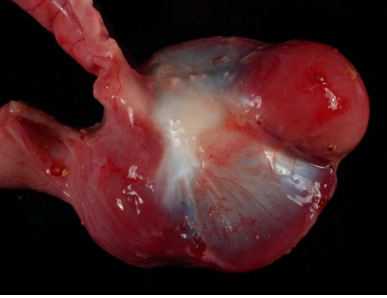
Unopened Gizzard
Gizzard unopened
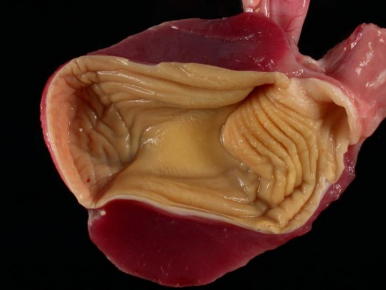
Gizzard Opened
Gizzard opened- note the koilin cuticle lining.
The gizzard consists mostly of two smooth muscle bands (thick and thin) that are oriented in different directions to create a powerful crushing action when they contract. The interior of the gizzard is lined with a thick protein cuticle (koilin) that protects the gizzard from 1) acid and proteolytic enzymes and 2) from injury during grinding of hard food substances. The koilin lining is often green from reflux of bile from duodenum into the gizzard. Grit (small stones) consumed and stored within the gizzard can increase digestibility of hard feeds (grains and seeds).
Gizzards of gamebirds with digestive tract disorders can distend with litter or particulate matter (abnormal feeding behavior from probable discomfort).
 Small intestine
Small intestine
The the intestine is lined by folds or villi with great absorptive capacity. Intestinal length varies in birds, but tends to be shorter on a body weight basis compared to mammals. This is where most of the food is enzymatically digested and absorbed.
- Duodenum- This organ has a duodenal loop that surrounds the pancreas. Has less digestive capacity than mammal duodenum because most digestive enzymes of birds empty into the end (distal aspect) of the duodenum near the jejunum. Most carbohydrate absorption occurs in the duodenum.
- Jejunum- Generally ends where the yolk stalk (Meckel’s diverticulum) is located. Major site of enzymatic digestion and absorption of amino acids, calcium and phosphorus.
- Ileum- shorter than jejunum and similar function, but on a slightly lower scale
Cecum
These are paired blind-ended sacs attached at the junction of the ileum and colon. Cecal contents are distinct from those of the small intestine and colon, having a slightly dry to tar-like consistency. The ceca are evacuated infrequently (several times a day) compared to the colon. Cecum serves as a site of fluid absorption (along with lower segments of intestine) and limited absorption of amino acids and carbohydrates.
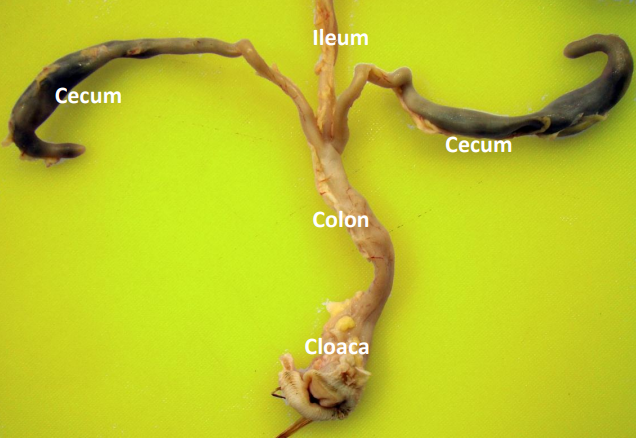
Pheasant Lower Digestive System
Liver of “normal” two-week pheasant chick
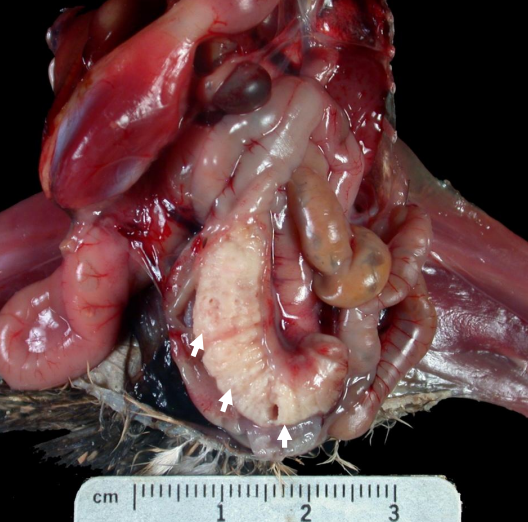
Cecal Coccidiosis
Three-week-old Chukar Partridge chick: Cecal coccidiosis- core of white exudate distends the cecum (arrows).
Three-week-old Chukar Partridge chick: Cecal coccidiosis- core of white exudate distends the cecum (arrows).
Colon and cloaca
The colon (rectum) is relatively short and links the ileum with the coprodeal compartment of the cloaca. The cloaca and colon are important for reabsorption of water from GI tract. The cloaca serves as a common end pathway for urinary tract (urates), digestive tract (feces) and reproductive tract. The colon empties into the most cranial portion, the coprodeum. The urinary tract and reproductive tracts empty into the middle and smallest compartment, the urodeum. The final compartment, the proctodeum opens to the outside (anus) and contains the bursa of Fabricius.
Pancreas
Reddish to tan tissue that lies within the loop of the duodenum. Releases insulin (endocrine function) into bloodstream and secretes amylase, lipase, proteolytic enzymes and sodium bicarbonate (exocrine function) into lumen of duodenum.
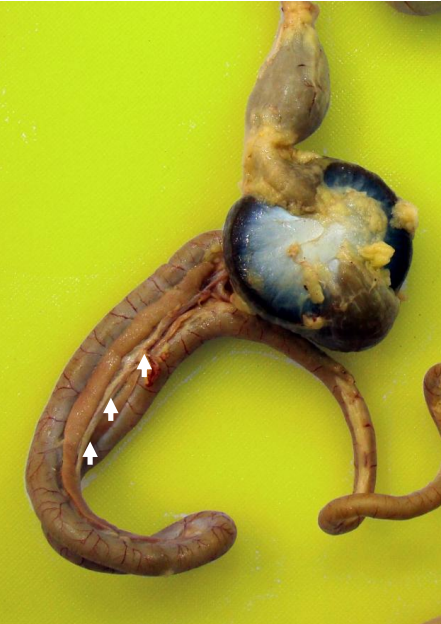
Liver
There are right and left lobes that are joined at the midline. The right lobe is largest in gallinaceous birds. Liver produces bile that is transported to the lumen of the duodenum by two ducts. Bile emulsifies lipid so that it can be more easily digested by lipase.
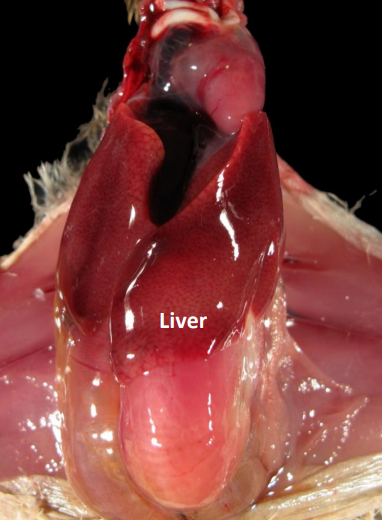
Liver of Chick
Liver of “normal” two-week pheasant chick
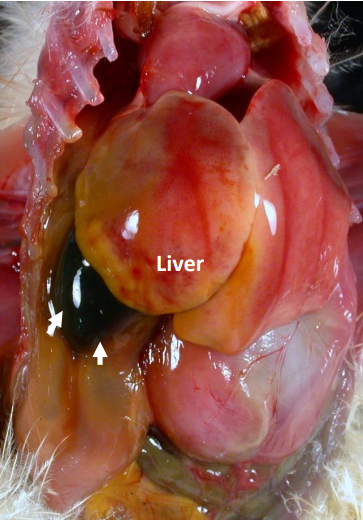
Liver of Chick
Liver of one-week pheasant chick with “starveout.” Note the enlarged gall bladder (arrows)
What does the liver do?
- Carbohydrate metabolism: maintaining constant blood glucose concentration
- Protein metabolism: synthesis of plasma proteins (such as albumin), coagulation factors (e.g. fibrinogen, prothrombin, factors V, VII, IX and X)
- Fat metabolism: hepatocellular up-take of surplus fatty acids bound to albumin (coming either from ingestion (from enterocytes) or from adipocytes) → use for energy production in hepatocytes plus synthesis of triglycerides
- Synthesize and excrete bile. Bile = bile acids, cholesterol, phospholipids, bilirubin, proteins plus mucoid secretions of gall bladder and bile duct glands, water and electrolytes.
- Bilirubin metabolism: end product of hemoglobin/myoglobin degradation during erythrocyte turn over in mononuclear phagocyte system (e.g. spleen, bone marrow and Kupffer cells of liver).
- Immune function (Kupffer cells, etc.) = Innate immune response: severe liver disease increases likelihood of endotoxemia and systemic infections.
- Biotransformation, and usually inactivation, of toxins
Where does absorption of nutrients occur?
- Carbohydrates: Absorption occurs more rapidly from the small intestine compared to the cecum. Most glucose absorption occurs in the duodenum by active transport.
- Amino acids and peptides: Most amino acid absorption occurs in the small intestine, although minimal absorption might occur in crop, gizzard and proventiculus (minimal).
- Fatty acids: Fatty acid and bile acid absorption occurs mostly in the jejunum and to a lesser extent in the ileum.
- Electrolytes: The upper jejunum is the major site of absorption of calcium, phosphorus and Mg.
- Water, sodium and chloride: Water is absorbed by the small and large intestines and ceca, and usually follows the absorption glucose, sodium and amino acids. Substantial absorption of sodium occurs in the jejunum and colon.
What are some of the common digestive tract diseases of gamebirds?
Candida albicans infection
- Definition: Mycotic infection affecting a wide variety of birds, primarily in the upper digestivetract. Yeast infection; fairly common, but generally not a major clinical problem
- Synonym: Candidiasis = crop mold = thrush
- Cause: Candida albicans; a yeast that forms pseudohyphae in tissues.
- Epidemiology/Transmission: Ubiquitous organism; overgrowth is usually controlled by normalbacterial microflora in digestive tract. Often associated with other disease problems in thehouse; digestive or respiratory tract disease. Long-term oral antibiotics, especially when administered in drinking water, can alter the microflora of the upper digestive tract and promote yeast growth. Not transmitted from bird to bird.
- Clinical signs/Gross lesions: Nonspecific; birds may appear unthrifty or have other more significant diseases being treated with oral antibiotics.
Lesions occur most often in the crop
Fine, white pseudomembrane lining oral cavity or esophagus. Membrane can be peeled off of mucosa- “Turkish towel” appearance to lining - Diagnosis: Gross and histopathological lesions are usually confirmatory, although fungal culture can also be considered.
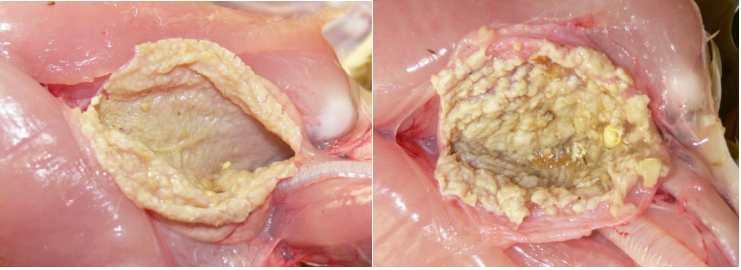
Candida Infection
Turkey poults with Candida infection. The photos above show moderate to severe crop infection(note the rough lining of the open crops) caused by Candida albicans.
Ulcerative Enteritis
This is a common bacterial enteric disease of domestic bobwhite quail, but also affecting other upland gamebirds, and young turkeys and white leghorn pullets. The lesions are characterized by multifocal discoid ulcers in the small intestine and multifocal hepatic necrosis.
Cause: Clostridium colinum, a gram-positive, spore-forming bacterial rod. The organism is hardy in the environment.
Transmission: The organism is spread in the feces of infected birds and can persist in the in the soil or litter for several months. The agent spreads rapidly from bird to bird. The infection can be spread in contaminated feces by flies. The disease is rare in birds raised on wire.
Clinical Signs: Quail are usually 6-10 weeks of age and sudden death associated with white, watery diarrhea occur. Birds that do not die suddenly will be depressed with closed eyes and ruffled feathers. Infected birds are thirsty and will huddle around the waterers. The course of disease is about two weeks and can result in nearly 100% mortality in bobwhite quail.
Gross lesions: Lesions are prominent in most affected birds. Crops may be distended with fluid. Affected birds have deep, punctate to discoid, tan to grey ulcers in the small intestine. The ulcers often penetrate the entire wall of the intestine to result in peritonitis and adherence of intestinal loops. The liver may or may not have pale foci of necrosis on the capsule and on cut surfaces. Birds that live for more than ten days can become emaciated.
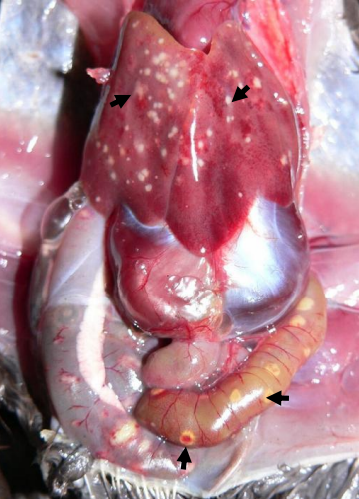
Quail With Ulcerative Enteritis
Four-week-old Bobwhite quail with ulcerative enteritis. Note the multifocal punctate ulcers in the small intestine and liver (arrows)
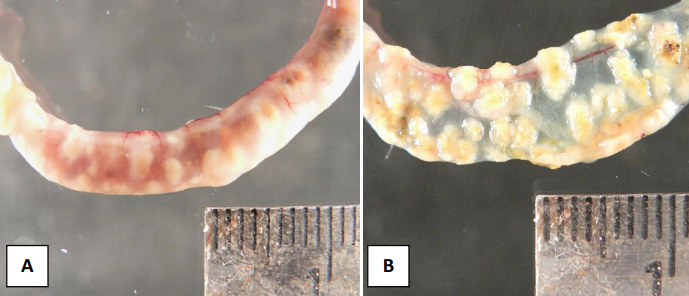
Quail With Ulcerative Enteritis
Small intestine of bobwhite quail with ulcerative enteritis. Note the multifocal punctuate transmural ulcers that are observed in the intact (A) and open (B) intestine.
Salmonellosis
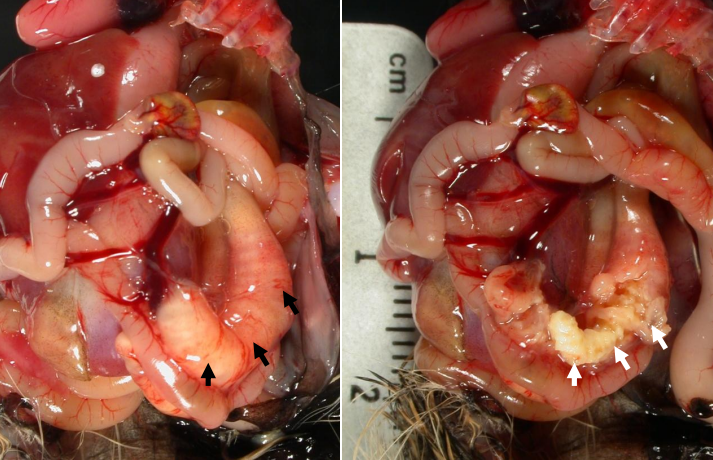
Pheasant Cecal Cores With Salmonellosis
This is infection with the Salmonella bacterium that most often causes disease of the digestive tract and yolk sac of gamebird chicks, but can also occur in adults with no clinical signs. In my experience Salmonella infection in gamebirds is primarily a chick problem, although the adults (breeders) can still be affected.
Cause: Salmonella pullorum-gallinarum as well as over 200 other serotypes (paratyphoid species) of Salmonella can be involved. S. pullorum-gallinarum has been described in a variety of upland game birds. S. pullorum-gallinarum is nonmotile and tends to be specific for birds, while the motile paratyphoid serotypes can infect a wide variety of birds and mammals. These are gram-negative bacterial rods.
Salmonella pullorum-gallinarum can be introduced into chicks by direct transmission from the ovary of the hen into the egg. Chicks can appear normal at hatch, but experience heavy mortality during the next three weeks- death in the early brooding stages.
The paratyphoid Salmonellae can enter the egg before hatch or infect the chick in the pen through a variety of routes, including:
- Soiled or manure-contaminated hatching eggs
- Contaminated by-products in feed
- Feces of other infected chicks
- Hatchery contamination
- Vectors: mice and wild birds
- Dirty litter or poorly disinfected flooring, feeders, drinkers
- Keeping young and old birds in close proximity
Diagnosis: Bacterial culture of sick birds (definitive) and blood testing (whole blood plate test) in adult breeder birds to identify reactors. The same killed Salmonella antigen is used to detect antibodies to both Salmonella pullorum and Salmonella gallinarum (fowl typhoid) in the whole blood plate test.Prevention/Treatment: Purchase birds only from NPIP-approved hatcheries. Salmonella pullorumgallinarum is a reportable disease. Birds will be euthanized, not treated under supervision of regulatory agency. Thoroughly disinfect the brooding pens prior to placing new chicks. Use clean, mold-free litter.Clinical Signs:Chicks will often appear as starve-outs, dead in pen, or piling in corners.
 Salmonella
Salmonella
The key to preventing or eliminating Salmonella infection is determining the source of the infection.
Paratyphoid infections can be treated with antibiotics, but affected chicks will probably die because they are usually not eating or drinking. (starve-outs). Antibiotics can help the birds in the pen that are least affected and most susceptible to contracting the infection.
- Diagnosis: History and gross lesions are usually diagnostic. The organism has specific growth requirements and bacterial isolated is generally not needed for a diagnosis.
- Treatment: Bacitracin is generally the antibiotic of choice at 100-200 gm/ton of feed for 7-10 days, or in the water at 0.25-0.50 gm per gallon of water for 7-10 days. Penicillin and tetracyclines can also be effective.
- Prevention: Ulcerative enteritis rarely occurs in birds that are raised on wire. Rotation of pens on a regular basis can reduce exposure to Clostridium spores.
References:
Aves Introduction, by AS King, In Sisson and Grossman’s The anatomy of Domestic Animals, Getty (ed.), Fifth Ed., W.B. Saunders Publishing, Philadelphia, PA, 1975.
Birds Their Structure and Function. AS King and J McClelland (eds.), Bailliere Tindall, London, 1977.
E R Dumont. Bone density and the lightweight skeletons of birds. Proc. Royal Soc. B , 277: 2193-2198, 2010.
Manual of Ornithology, Avian Structure and Function. NS Procor and PJ Lynch (eds.) Edwards Brothers, Inc. Ann Arbor, MI, 1993
Ornithology in Laboratory and Field, Fourth Edition, O S Pettingill, Jr. (ed.), Burgess Publishing Co., Minneapolis, MN, 1975.
Scott’s Nutrition of the Chicken, 4th Edition. S Leeson and JD Summers. University Books, Guelph, Ontario Canada, 2001.
Sturkie’s Avian Physiology, Fifth Edition. GC Causey (ed.). Academic Press, San Diego, CA. 2000.


